fe 3+ configuration|Fe, Fe2+ & fe3+ Electron Configuration(Read This First) : Pilipinas In writing the electron configuration for nitrogen the first two electrons will go in . ɪt, æl- / .
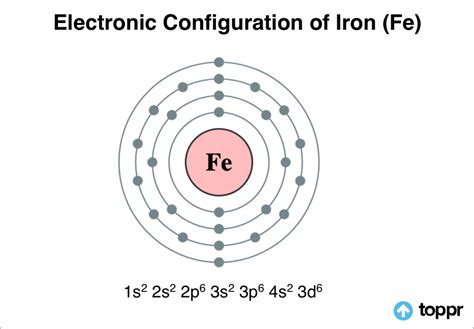
fe 3+ configuration,Electron Configuration for Iron (Fe, Fe2+, and Fe3+) Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period .
Iron (Fe, Fe 2+, Fe 3+) Read my article in Science Education based on my .In order to write the Na electron configuration we first need to know the .In writing the electron configuration for nitrogen the first two electrons will go in .
In order to write the Mg electron configuration we first need to know the .Iron (Fe, Fe 2+, Fe 3+) Read my article in Science Education based on my .
The next six electrons will go in the 2p orbital. The p orbital can hold up to six .
In order to write the Argon electron configuration we first need to know the .How to Write the Electron Configuration for Oxygen. Oxygen is the eighth element .

To write the configuration for the Iron ions, first we need to write the electron configuration for just Iron (Fe). We first need to find the number of elec.Iron exhibits these three allotropic forms at different temperatures when it cools down to molten form. The electronic configuration of Fe 2+ is 1s . Chemistry Electron Configuration Electron Configuration. 1 Answer. anor277. Jun 21, 2016. For F e, Z = 26; there are thus 23 electrons to distribute for F . Electron Configuration of Fe2+ and Fe3+. chemistNATE. 266K subscribers. Subscribed. 5.3K. 705K views 10 years ago. The electron configuration for Fe2+ is 1s2 2s2 2p6 . This chemistry video tutorial explains how to find the electron configuration of Ions such as Mg2+, P3-, Fe2+, and Fe3+.Electron Configuration - Basic Intro:.3.1: Electron Configurations. Page ID. Skills to Develop. Derive the predicted ground-state electron configurations of atoms. Identify and explain exceptions to predicted electron configurations for atoms and ions. . Fe has the electron configuration [Ar] 3d^6 4s^2, Fe2+ has the electron configuration [Ar] 3d^6, and Fe3+ has the electron configuration [Ar] 3d^5. The .The electron configuration of Fe 3+ ion can be determined by removing three electrons from the Fe atom. The atomic number of Fe is 26, indicating that a neutral Fe atom has .Structure of the octahedral ferricyanide anion. Because the overall charge of the complex is 3-, Fe is in the +3 oxidation state and its electron count is 3d 5. The same procedure can be applied to any transition metal complex. For example, consider the complex [Cu (NH 3) 4] 2+. Because ammonia is a neutral ligand, Cu is in the 2+ oxidation . Quando 3 elétrons são removidos do átomo de fe neutro, o íon fe3+ é formado. O gás nobre fe3+ configuração eletrônica é 1s2 2s2 2p6 3s2 3p6 3d5. 1º 2 elétrons são removidos do orbital 4s porque ele tem energia mais alta que o orbital 3d e então 1 elétron removido do orbital 3d fazendo com que a configuração eletrônica da . The electron configuration for Fe2+ is 1s2 2s2 2p6 3s2 3p6 3d6The electron configuration for Fe3+ is 1s2 2s2 2p6 3s2 3p6 3d5Ask me questions: http://www.chem.What is the electron configuration of: (a) Na + (b) P 3– (c) Al 2+ (d) Fe 2+ (e) Sm 3+ Solution First, write out the electron configuration for each parent atom. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it, but listing the core-abbreviated electron configurations is also .
fe 3+ configuration The Electronic Configuration of Iron. A chemical element with an atomic number 26 and a symbol Fe represents it, Iron is a very common element that is found. Unlike other elements, iron exists at the oxidation states of -2 to +6. Elementary iron occurs in a low-oxygen environment in spite of being reactive to water and oxygen. A Ferric ion or Fe 3+ is a trivalent metal cation and a monoatomic trication. It is the oxidized form of iron (Fe) metal. . When three electrons are lost from an iron atom it forms a ferric ion or Fe 3+. The electronic configuration is as follows, Fe = 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. and Fe 3+ = 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5. The electron configuration of $\ce{Fe(III)}$ is $1s^22s^22p^6 3s^23p^6 3d^5$. The half filled orbital $3d$ is more stable than the same orbital filled with 6 electrons. So, $\ce{Fe(III)} $ ion is more common than $\ce{Fe(II)}$. Share. . The electron configuration for the iron (III) ion is: 1s2s22p63s23p63d5. The element iron, Fe, has the atomic number 26, which is the number of protons in its atomic nuclei. A neutral iron atom has 26 protons and 26 electrons. In order to form a 3+ ion, it must lose three electrons.View this answer. The electron configuration for the Fe3+ ion is 1s2 2s2 2p6 3s2 3p6 3d5. Normally, a neutral atom of iron would have two electrons in the 4s sublevel. See full answer below.[F e F 6] 3 − has Fe atom _____ hybridised with unpaired _____ electrons. d 2 s p 3, 4; d 2 s p 3, 5; s p 3 d 2, 5; s p 3 d 2, 3; A. d 2 s p 3, 4. B. d 2 s p 3, 5. C. s p 3 d 2, 5. D. s p 3 d 2, 3. Open in App. Solution. Verified by Toppr [F e F 6] 3 −, F e + 3, d 5 configuration, high spin complex so it have 5 unpaired electron & s p 3 d 2 . Electron Configuration Chart. * note the irregularity. You may also view the electron configurations of the elements on a printable periodic table if desired. Look up the electron configuration of any element using .fe 3+ configuration Fe, Fe2+ & fe3+ Electron Configuration(Read This First) Compounds in which all of the electrons are paired are diamagnetic. Technically, they are repelled by the poles of a magnet, but this repulsion is usually too small to notice. Paramagnetic compounds contain one or more unpaired electrons and are attracted to the poles of a magnet. Elemental iron and iron (III) are paramagnetic . P 3– Al 2 + Fe 2 + Sm 3 + Solution. First, write out the electron configuration for each parent atom. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it, but listing the core-abbreviated electron configurations is also acceptable. Next, determine whether an .Fe, Fe2+ & fe3+ Electron Configuration(Read This First) So far, we have studied the electron configuration for elements in periods 1-3 on the periodic table in which we filled s and p orbitals. When we get to period 4-7 on the periodic table, . The element is iron, Fe. b) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 7. The element is Rhodium, Rh.

In the case of first row transition metals, the electron configuration would simply be [Ar] 4s x 3d x. The energy level, "n", can be determined based on the periodic table, simply by looking at the row number in which the element is in. However, there is an exception for the d-block and f-block, in which the energy level, "n" for the d block is .What is the electron configuration of Fe 3+?. The electron configuration of Fe 2+ is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5.Fe 3+ atom loses four more valence electrons from its d-orbital to attain noble gas configuration.. What is the complete electron configuration of Fe 3+?. The complete electron configuration of Fe 3+ is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5.Fe 2+ .
Write electronic configuration of `Fe^(2+)` and `Fe^(3+)` ions. Which of these has more number of unpaired electrons? Atomic no. of `Fe` is `26`. Characteristic reactions of Fe²⁺ and Fe³⁺. The [Fe(H2O)6]3+ [ Fe ( H 2 O) 6] 3 + ion is colorless (or pale pink), but many solutions containing this ion are yellow or amber-colored because of hydrolysis. Iron in both .
fe 3+ configuration|Fe, Fe2+ & fe3+ Electron Configuration(Read This First)
PH0 · What is the electron configuration for #Fe^(3+)#?
PH1 · Unveiling the orbital diagram of Fe 3+
PH2 · Fe, Fe2+ & fe3+ Electron Configuration(Read This First)
PH3 · Fe, Fe2+ & fe3+ Electron Configuration(Read This First)
PH4 · Electronic Configuration of Iron
PH5 · Electron Configuration of Ions
PH6 · Electron Configuration of Fe2+ and Fe3+
PH7 · Electron Configuration for Iron (Fe, Fe2+, and Fe3+)
PH8 · Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions)
PH9 · Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions)
PH10 · Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions)
PH11 · 3.1: Electron Configurations